Trans Dichlorobis Ethylenediamine Platinum Iv
 | |
 | |
| Names | |
|---|---|
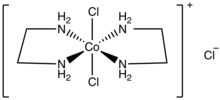
| IUPAC proper noun (OC-6-12′)-Dichloridobis(ethane-1,2-diamine-κ2 N,N′)cobalt(1+) chloride(i−) | |
| Identifiers | |
| CAS Number |
|
| 3D model (JSmol) |
|
| PubChem CID |
|
| InChI
| |
| SMILES
| |
| Properties | |
| Chemical formula | C4H16ClthreeCoNiv |
| Molar mass | 285.48 grand·mol−1 |
| Advent | green solid |
| Melting point | decomposes |
| Solubility in water | practiced |
| Hazards | |
| GHS labelling: | |
| Pictograms |  |
| Signal discussion | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P261, P305+P351+P338 |
| Except where otherwise noted, data are given for materials in their standard land (at 25 °C [77 °F], 100 kPa). Infobox references | |
trans-Dichlorobis(ethylenediamine)cobalt(3) chloride is a salt with the formula [CoCl2(en)ii]Cl (en = ethylenediamine). It is a green diamagnetic solid that is soluble in h2o. It is the monochloride salt of the cationic coordination complex [CoClii(en)2]+. One chloride ion in this salt readily undergoes ion exchange but the two other chlorides are less reactive, being spring to the metal center. The more stable trans-dichlorobis(ethylenediamine)cobalt(III) chloride is also known.
Synthesis [edit]
The compound is synthesized by the reaction of cobalt(II) chloride and ethylenediamine in muriatic acid in the presence of oxygen:
- 4 CoCl2 + eight en + 4 HCl + Otwo → 4 trans-[CoCltwo(en)2]Cl + 2 HiiO
The initial production contains HCl, which is removed past heating. Alternatively, (carbonato)bis(ethylenediamine)cobalt(III) chloride reacts with muriatic acid at 10 °C to give the same species.[1]
- [Co(CO3)(en)2]Cl + two HCl → trans-[CoCl2(en)2]Cl + COtwo + H2O
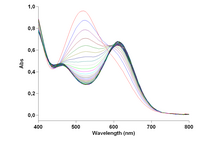
UV-vis spectra of diverse stages in the conversion of trans-[CoCl2(en)2]+ to the cis isomer.
Comparison of cis and trans isomers [edit]
This common salt is more soluble than the cis isomer. This pair of isomers was pregnant in the evolution of the surface area of coordination chemistry.[2]
The trans isomer cation has idealized D2h point group symmetry, whereas the cis isomer cation has C2 symmetry.
References [edit]
- ^ Springbørg, J.; Schaffer, C. E. (1973). "Dianionobis(Ethylenediamine)Cobalt(III) Complexes". Inorganic Syntheses. Inorganic Syntheses. Vol. fourteen. p. 63-77. doi:x.1002/9780470132456.ch14. ISBN9780470132456.
- ^ Jörgensen, S.M. "Ueber Metalldiaminverbindungen" Journal für praktische Chemie (in German language), 1889, book 39, page viii. doi:ten.1002/prac.18890390101
Trans Dichlorobis Ethylenediamine Platinum Iv,
Source: https://en.wikipedia.org/wiki/Trans-Dichlorobis(ethylenediamine)cobalt(III)_chloride
Posted by: wyattblem1987.blogspot.com


0 Response to "Trans Dichlorobis Ethylenediamine Platinum Iv"
Post a Comment